Minimal Residues: Process and Product Risk
Reproducible, validated, and MDR-compliant Cleaning for Medical Technology
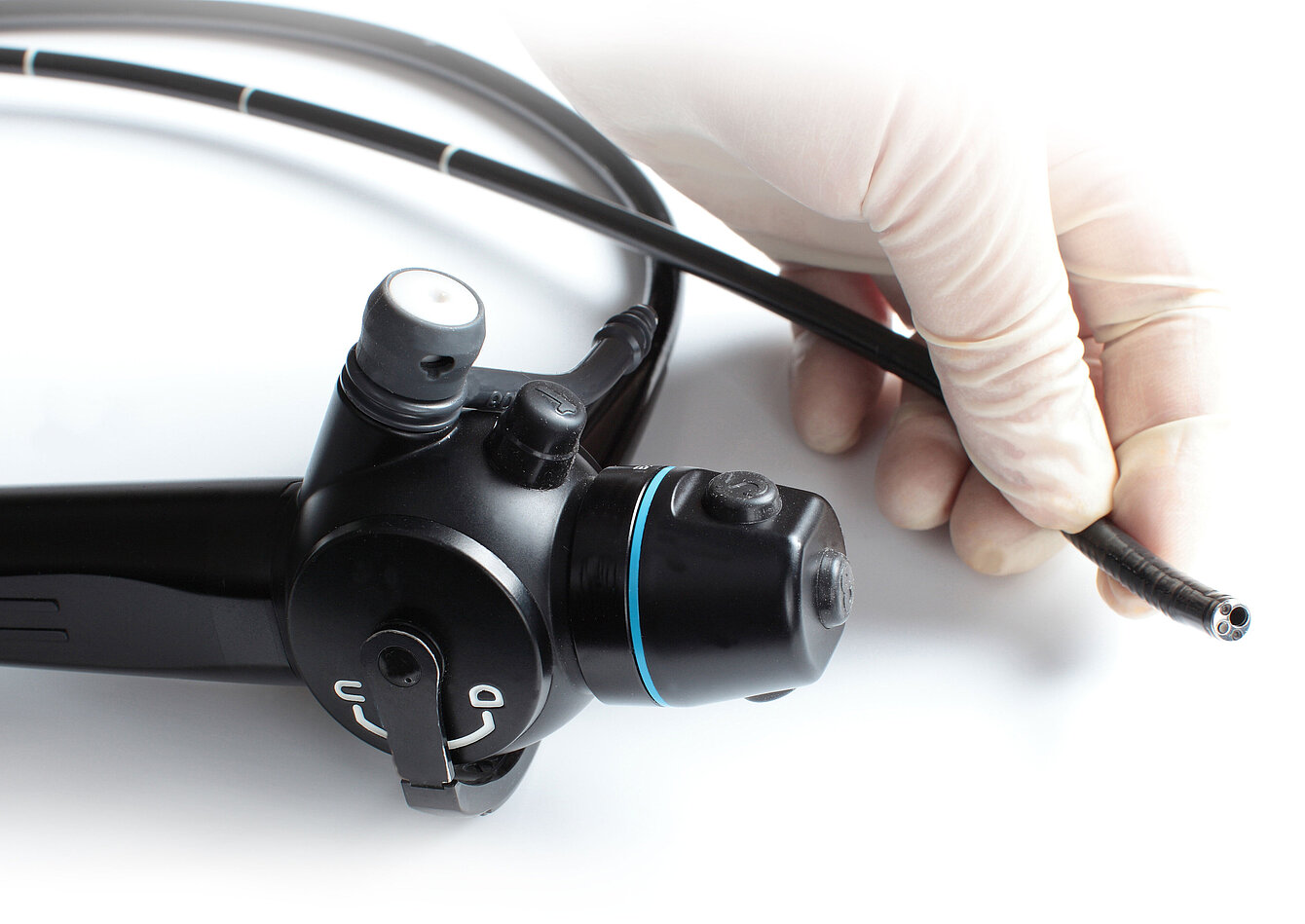
Manufacturing at the Highest Level – with Cleaning as a Challenge
The established manufacturer, headquartered in Baden-Württemberg – Germany’s largest pharma and MedTech hub – develops and produces exclusively in-house, with a manufacturing depth that extends from raw material to final assemblies. The portfolio includes delicate metal parts, complex forming and stamping technology, and precision mechanical components. Surgical stainless steels and other medical-grade alloys are processed with tolerances in the micrometer range.
Production is highly automated, workpiece-guided and digitally traceable to meet stringent national and international quality standards. While cleanability is already considered during design, the removal of filmic and particulate residues remains a critical step. The question: how can this process be designed to guarantee technical cleanliness at a consistently high level – across series production, product variants, and customer-specific solutions alike?
Cleaning between Reality and Expectation
Many components – especially for endoscopy – feature capillary structures, cavities, or narrow lumens. These areas cannot be sufficiently cleaned with conventional ultrasonic immersion or spray systems, making manual rework necessary. Sensitive geometries are often cleaned only semi-automatically or entirely by hand. The result: high personnel costs and cleaning quality dependent on the operator. An audit-proof, reproducible, and documentable process in accordance with MDR or ISO 13485 is virtually impossible. Testing effort increases – and in the worst case, approval is delayed.

Dedicated cleaning stations for individual parts are no sustainable alternative. Long setup times drive up unit costs, and flexibility drops to zero. Moreover, regardless of the method, cleaning results are often verified only after the fact – through contact tests, visual inspection, or laboratory analysis. If deviations are discovered at this stage, entire batches may have to be re-cleaned.
"Structured approach, strong technical expertise, a genuine partnership – and a result that convinces us every day. Cleaning is no longer a secondary process, but an integral part of our MDR-compliant process chain."
Customer Feedback
From variable Results to validated Processes
Faced with these challenges, it became clear: a new cleaning solution was needed. No more improvised, operator-dependent processes. Instead of manual effort and rework, cleaning should be stable, automated, and fully validatable – an integral step in manufacturing. The goals: lower costs, shorter throughput times, and smooth regulatory approval.
The first Step to the best Solution: Industry and Process Understanding
The start of collaboration with MAFAC? For the customer, unusual. For the cleaning experts, standard practice. Instead of presentations, the process begins with questions: Which geometries cause increased cleaning effort? When and where do residues occur – and what kind? On the one hand particulate contamination from machining, lodging in fine structures and lumens. On the other filmic residues from forming lubricants or corrosion protection, invisible but bioactive and potential carriers of germs.
And, also, chemical residues from process chemistry or detergents, which may be irritant or even toxic. And finally: which test methods are currently used – and how can they be improved?
Together, answers are developed: drawings and samples are analyzed, critical zones identified, and requirements for cleaning, drying, and documentation defined in detail. Only when all questions are resolved and the requirements matrix is complete does the solution emerge – not as a standalone system, but as an integrated process.
"Every project in medical technology – and beyond – starts for us with listening and understanding. Only those who truly know the customer’s processes can develop a cleaning solution that stands the test of time – technically, regulatory, and economically. For us, that’s not an added service – it’s our standard."
Kai Klussmann, Head of Product Management at MAFAC

Cleaning That Reliably Masters Variety – Today and Tomorrow
The physical core for cleaning and drying is a MAFAC cleaning system with a closed process chamber, utilizing MAFAC Vector Kinematics and MAFAC VAP (Vacuum Activated Purification). The pressure-change technology works through specific patterns of negative and positive pressure change in the cleaning bath. Thus, creating currents that efficiently penetrate deep lumens and shadowed areas.
Automated, workpiece-guided processes, precisely controllable parameters – temperature, pressure, media quality, drying level – and digital interfaces make the process both flexible and reproducible, with full documentation. Different geometries and contamination scenarios can be handled within one system, without constant retooling.
In place of the previously inconsistent and partly insecure methods now stands a stable, validatable, and scalable process – one that reliably meets all regulatory requirements and integrates seamlessly into QS and traceability structures.
Stability in day-to-day Production, Strategy for Tomorrow
Since integrating the new cleaning solution, the customer has achieved norm-compliant technical cleanliness even in complex internal geometries – safely and reproducibly. Manual rework has been eliminated, freeing qualified personnel for higher-value tasks. Cleaning is no longer a cost unit driver, but a factor for greater efficiency. The entire process is documented, auditable, and MDR-compliant. A solid foundation on which the company is now establishing a consistent cleaning strategy across its entire product portfolio.

The final word on MAFAC:
This project once again demonstrates that it is not about selling a system, but about meeting the claim of being an “Architect of Cleanliness”. With industry knowledge, process expertise, and patented technologies that master even the most demanding tasks in medical technology.







