Validatable Cleaning in Medical Technology
You manufacture or supply medical components and products that must be clean, fully documented
and MDR-compliant throughout every step of production.
MAFAC develops a cleaning process that integrates seamlessly, reliably removes all typical contamination types –
from filmic to bioburden – and adapts precisely to new requirements or component variants.
Sovereign Solutions for demanding MedTech Requirements:
| • Clean complex geometries reliably. With deep action in lumens, capillaries and shaded areas. |
| • Enable validated and audit-proof process control. With fully documented and verifiable parameters. |
| • Handle variants, batch sizes and new products with confidence. With consistent results across component changes and series production. |
| • Achieve targeted drying. Absolutely dry surfaces and internal structures, ready for downstream processes and logistics. |
| • Ensure the quality of downstream processes. Technical cleanliness for coating, assembly, sterilization or packaging. |
| • Comply with relevant purity standards. For example VDA 19, ISO 16232 or ISO 10993. |
A to Z: The full Spectrum
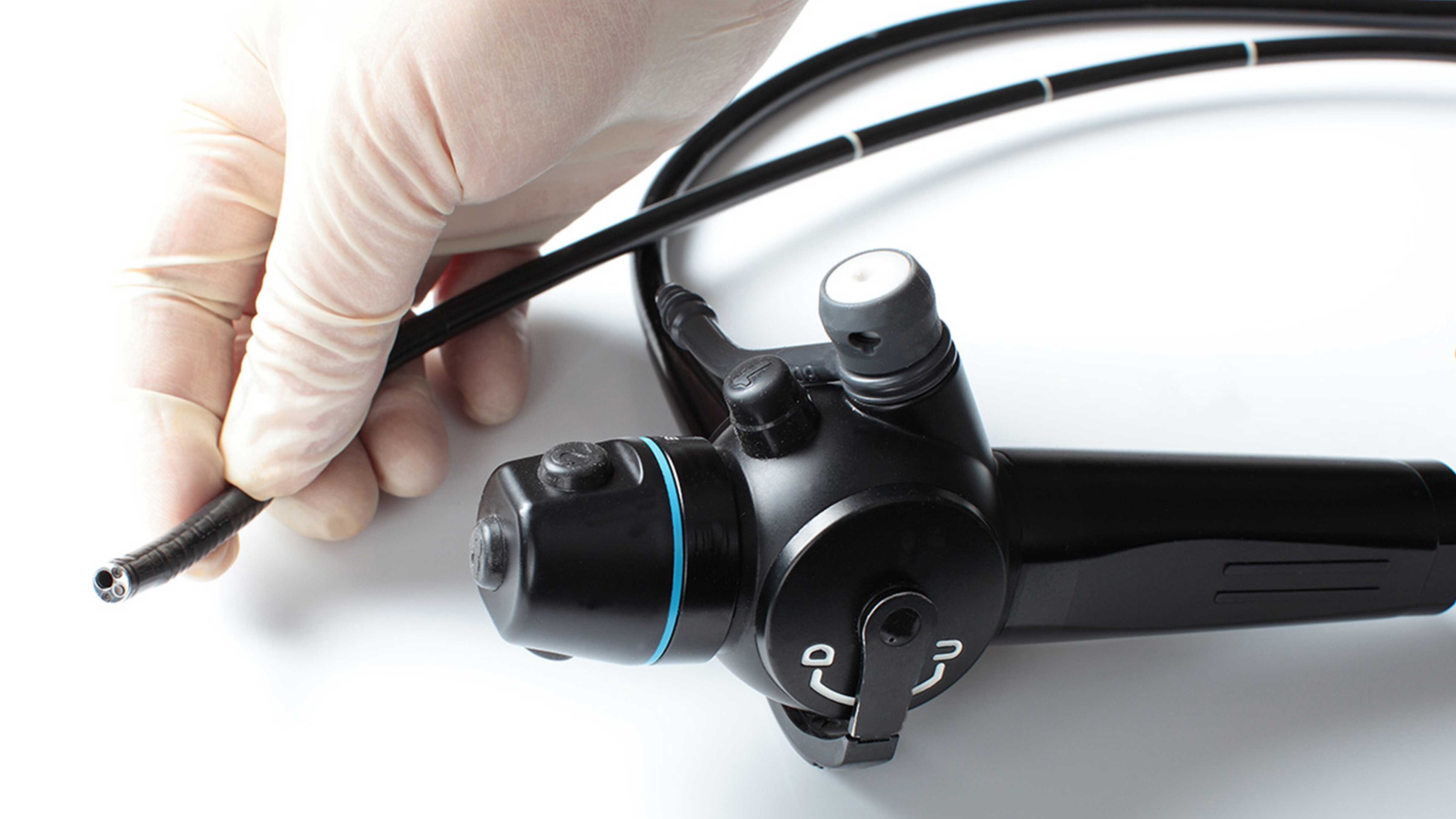
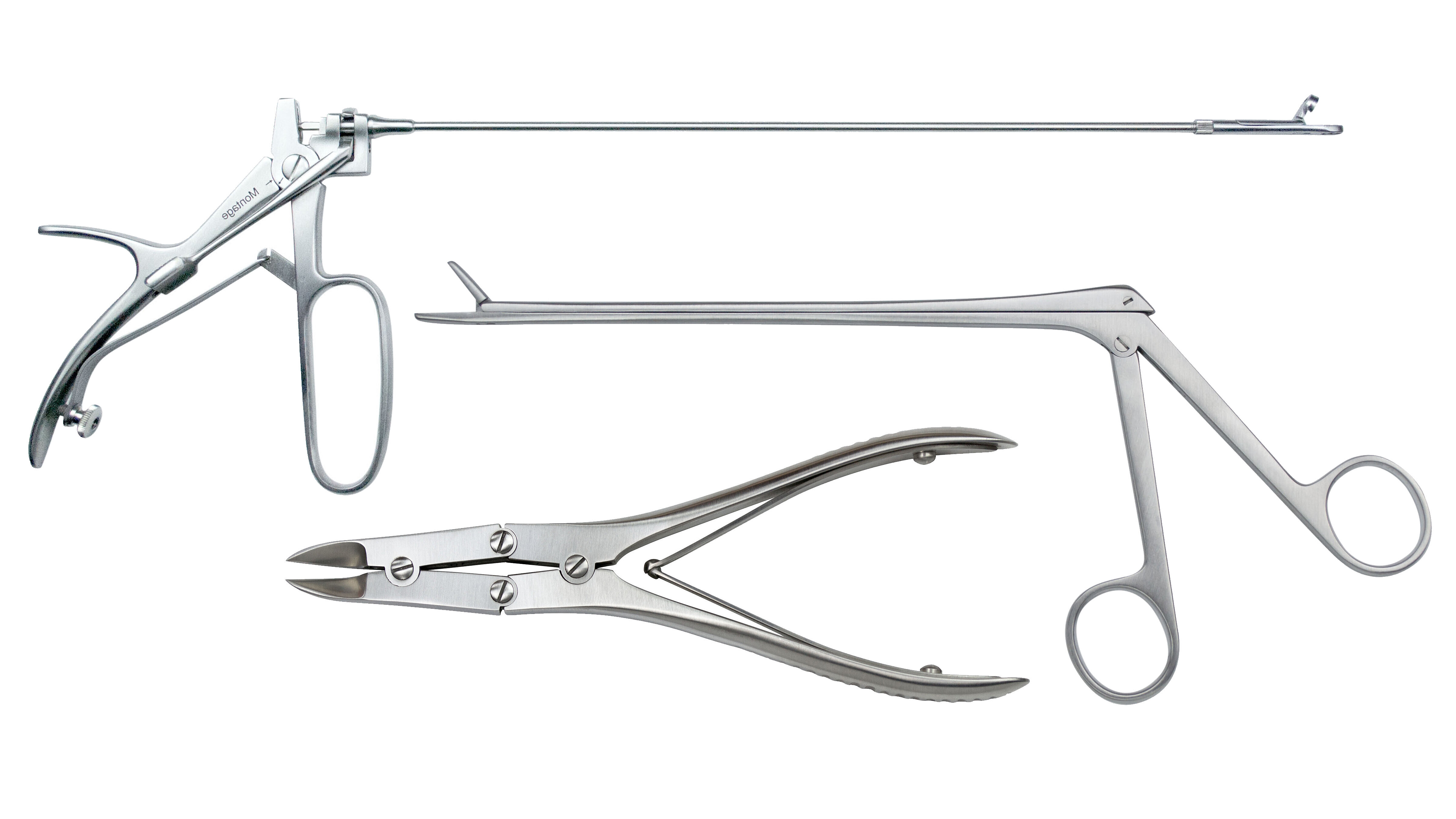
Whether surgical instruments or endoscopy components, subassemblies for devices such as syringes or injectors, measuring instruments or high purity endoprostheses: we ensure reproducible cleanliness across all categories and prepare your products for downstream process steps.
>> view application examples
Understanding processes.
Shaping cleanliness. Medical technology requires processes that ensure the highest quality at every step. This applies to your entire production – and equally to cleaning. Whether pre-cleaning after machining, intermediate cleaning as the basis for downstream steps such as coating, or final cleaning with complete drying: MAFAC develops the cleaning solution that fits your requirements. To do so, we contribute our expertise in demanding industries and sensitive applications from the very beginning.
We analyze your components, identify typical contamination patterns and define the cleaning strategy. On this basis – and considering further criteria such as batch sizes, applicable standards and required performance – we select the appropriate cleaning system from our portfolio and the right combination of our patented technologies. The result is a process that achieves reproducible technical cleanliness and integrates seamlessly into your production structure – today and for future tasks.
MAFAC Cleaning Competence for MedTech
Reproducible cleanliness for sensitive products is achieved at MAFAC using water-based, solvent-free
processes – built on our patented technologies and precisely aligned with your requirement profile.
>> see technical highlights of your industry solution
Fulfilling Cleanliness Requirements with verifiable Precision
Our cleaning solutions ensure that you fulfil all relevant cleanliness standards and industry-specific requirements in an audit-proof manner.
We can integrate your internal test procedures and document the required cleanliness – for valid, traceable process control.
| Contaminations | Standards |
|---|---|
| Particulate contamination | VDA 19, ISO 16232, ASTM F2459-12 |
| Filmic contamination | ISO 10993-18, USP <643>, ASTM F2847-10 |
| Organic residues | ISO 10993-18, USP <643>, ASTM F2847-10 |
| Cytotoxicity | DIN EN ISO 10993-5, USP 87 |
| Bioburden | ISO 11737-1 |
| Endotoxins | DIN EN ISO 11737-3, USP <85> |
| Ionic contamination | ISO 7888, USP <645> |
Can a cleaning process be validated in compliance with the MDR?
Yes, of course. Validation requires reproducible processes and measurable parameters. We document all variables – pressure, temperature, media quality and time. Test cleanings define the parameters, and multiple batches demonstrate process stability. The process is traceable and auditable in accordance with the MDR and ISO 13485; if required, we jointly prepare the requirements matrix.
What role does component geometry play in medical device cleanability?
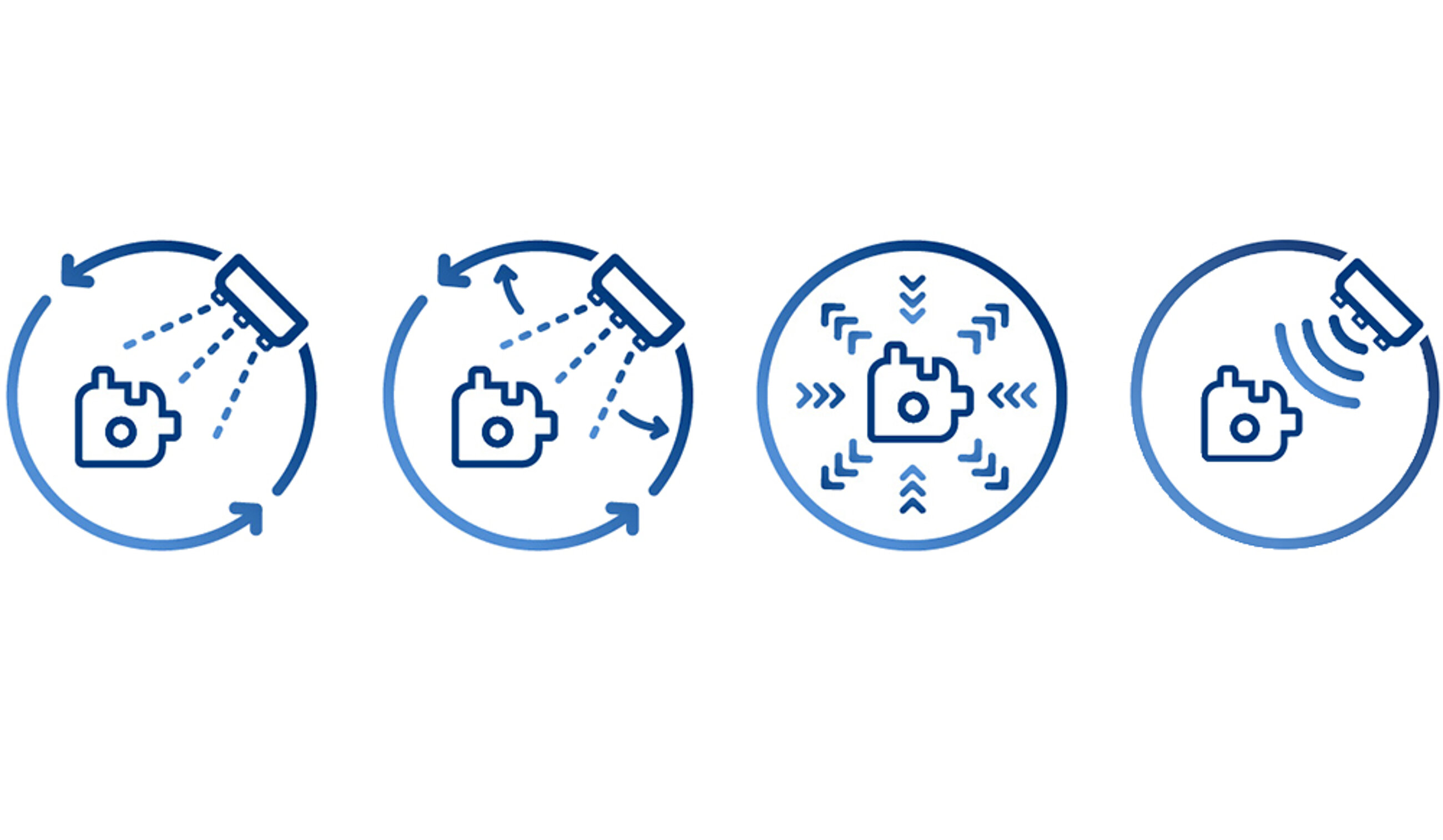
Lumens, cavities and capillary structures are difficult to access and therefore critical. Shadowed areas are best reached through directed flow and pressure variation. Filigree mechanisms such as joints or springs pose a risk of residual contamination; internal channels – for example in endoscopic components – are typical challenge zones. MAFAC Vectorkinematics and MAFAC VAP are designed for complex geometries and reliably remove both filmic and particulate residues.
Can MAFAC integrate customer-specific test procedures?
Yes. We can integrate particulate residual contamination analysis directly into the process. Tests such as TOC, cytotoxicity or chemical analyses are performed downstream, outside the system. Our processes can be aligned with OEM-specific requirements as well as with standards such as VDA 19, ISO 16232 or ISO 10993-18.
What are the advantages of combining the MAFAC VAP pressure-change technology with rotating ultrasonics?
The MAFAC VAP pressure-change technology penetrates deeply into lumens and capillaries, while MAFAC rotating ultrasonics generates uniform cavitation. The combination reliably reaches complex internal structures and removes both particulate and filmic residues. This ensures high cleaning quality even for demanding components, e. g. in surgery or endoscopy.
Does MAFAC also comply with company-specific standards and requirements?
Yes. We tailor processes beyond general regulations to meet company-specific limits and test methods, with part-specific programs reproducibly stored in the system. Freely adjustable parameters allow individual specifications; documentation and validation are also possible in line with your internal quality systems.